





PURE SUBSTANCE |
MIXTURE |
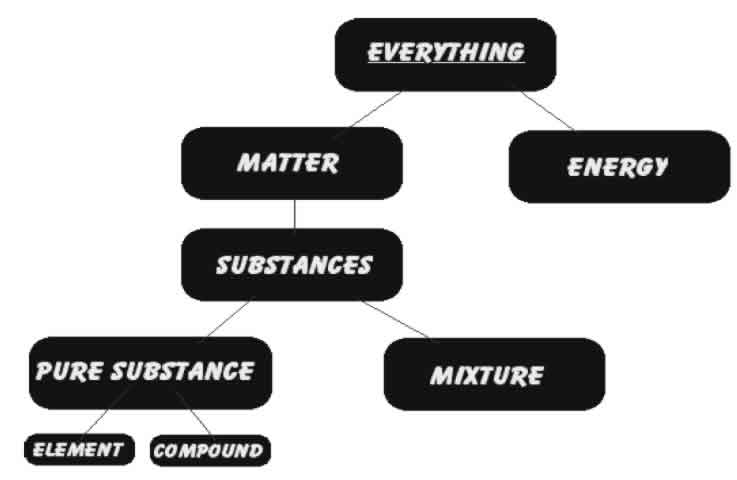
| • Pure substance consists of a single type of substance . | • Mixture consists of two or more pure substances. |
| • Pure substance cannot be separated into other substances by physical methods | • Mixture can be separated into its components by physical methods. |
| • Pure substance has its own definite properties | • Mixture shows the properties of its components. |
Elements are made up of one kind of atoms only. Compounds are made up of one kind of molecules only.
Q.1 Is air around us a compound or mixture?
Q.2 Water is a compound. Justify.
Q.3 Classify the following as element , compound and mixture: Iron , sea water , Milk
Q.4 Are the naturally occurring material in nature chemically pure substances?
i) On the basis of their physical states:
SOLID
SOLID |
LIQUID |
GAS |
|
SOLID |
• Salt and sugar | • Salt and water | • Dust in air |
| LIQUID | • Mercury and copper | • Alcohol and water | • Clouds |
| GAS | • Hydrogen and palladium | • Oxygen and water | • Air |
ii) On the basis of miscibility:
Homogeneous Mixture |
Heterogeneous Mixture |
| • It consists of single phase. • Uniform composition. • Example: Sugar dissolved in water
|
• It consists of two or more phase. • Does not have uniform composition. • Example: Air, sand and common salt.
|
Q.1 Give one example for each of the following mixtures:
i) Solid/solid (homogeneous)
ii) Solid/solid (heterogeneous)
iii) Liquid/liquid (homogeneous)
iv)Liquid/liquid (heterogeneous)
v) Gas/liquid (homogeneous)..
Q.2 Classify the following as homogeneous & heterogeneous mixture:
i) sodium chloride & water
ii) glucose & water
iii) sand & water
iv) air
4. Separating the components of a mixture
The components of a heterogeneous mixture can be separated by
1.Simple methods like :-
hand picking , sieving , & Winnowing
2.Special techniques like: -
i) Evaporation : a mixture of salt and water or sugar and water.
ii) Centrifugation : Butter from curd, Fine mud particles suspended in water.
iii) Decantation (Using separating funnel) : Oil from water.
iv) Sublimation : Camphor from salt,
v) Chromatography : Different pigments from an extract of flower petals.
vi) Distillation and fractional distillation : Separating components of Petroleum
vii) Magnetic separation: Iron pins from sand.
Q.1 Name the process you would use to :
i) recover sugar from an aqueous sugar solution.
ii) separate mixture of salt solution and sand.
Q.2 How will you separate a mixture of sand , water and mustard oil ?
5. Concentration of Solution
The amount of solute present in a given amount (mass or volume) of solution.
Concentration of a solution = Amount of solute / amount of solvent
The concentration of a solution can be expressed as mass by mass percentage or as mass by volume percentage.
Mass by mass percentage of a solution = Mass of solute × 100/ Mass of solution
Mass by volume percentage of a solution = Mass of solute ×100/Volume of solution
a) on the basis of size of solute particles:
True solution |
Sol [ Colloid] |
Suspension |
| • Homogeneous | • Heterogeneous | • Heterogeneous |
| • Size of solute particles is less than 1 n m or 10-9 m . | • Size of solute particles is between 1 nm to 1000 nm. | • Size of solute particles is more than 1000 nm. |
| • Particles cannot pass through filter paper | • Particles can pass through filter paper. | • Particles cannot pass thorough filter paper. |
| • Stable | • Stable and settle only on centrifugation | • Unstable and settle down on its own. |
| • Solution of sodium chloride in water, sugar & water. | • Milk , Fog | • muddy water, chalk & water, • smoke in the air. |
Colloidal solution is a heterogeneous mixture. It consists of two phases:-
(i) Dispersed phase : component present in small proportion
(ii) Dispersion medium : component present in large proportion
The particles of colloid are large enough to scatter a beam of light passing through it and make its path visible. Thus, they show Tyndall effect.
The colloidal particles are moving at random in a zigzag motion in all directions. This type
of zig-zag motion of colloidal particles is called Brownian movement.
b) On the basis of amount of solute:
|
Unsaturated solution |
Saturated Solution |
Supersaturated solution |
| A solution which has lesser amount of solute that it can dissolve at a given temperature is known as unsaturated solution. | A solution which has maximum amount of solute that it can dissolve at a given temperature is known as saturated solution. | A solution which can dissolve amount of solute by increasing temperature saturated solution is known as supersaturated solution. |
c) On the basis of nature of solvent
|
Aqueous solution |
Non-Aqueous solution |
| The solution in which the solvent is water is known as aqueous solution. | The solution in which the solvent is other than water (ether, alcohol or aceton) known as non-aqueous solution. |
Q.1 Classify the following substances into true solutions and colloidal solutions. Milk , ink , starch dissolved in water.
Q.2 A solution has been prepared by dissolving 5g of urea in 95 g of water. What is the mass percent of urea in the solution?
Q.3 Give an example of an aqueous solution in which gas is dissolved.
Changes that do not result in the production of a new substance.
• If you melt a block of ice, you still have H2O at the end of the change.
• If you break a bottle, you still have glass
Examples : melting, freezing, condensing, breaking, crushing, cutting, and bending.
- Changes that result in the production of another substance.
• As in the case of autumn leaves, a change in color is a clue to indicate a chemical change.
• a half eaten apple that turns brown.
Q.1 Which of the following is an example of physical change?
a. Mixing baking soda and vinegar together, and this causes bubbles and foam.
b. A glass cup falls from the counter and shatters on the ground.
c. Lighting a piece of paper on fire and the paper burns up and leaves ashes.
d. Baking a birthday cake for your mother.
Q.2. Which of the following is an example of chemical change?
a. Filling up a balloon with hot air.
b. Taking a glass of water and freezing it by placing it in the freezer.
c. A plant collecting sunlight and turning it into food.
d. Your dog ripping up your homework.
Q3. Which change can be easily be reversed?
a. Chemical Change
b. Physical Change
c. Both a physical and chemical change
d. Neither a physical or chemical change
7.Alloys
A material that has metallic properties and is composed of two or more chemical elements of which at least one is a metal .
• These cannot be separated into their components by physical methods.
• However, these are considered as mixture because these show the properties of its
constituents and can have variable composition.
The benefit of alloys is that you can combine metals that have varying characteristics to create an end product that is stronger, more flexible, or otherwise desirable to manufacturers.
• Aluminium alloys are extensively used in the production of automotive engine parts.
• Copper alloys have excellent electrical and thermal performance, good corrosion
resistance, high ductility and relatively low cost.
• Stainless steel alloys are used for many commercial applications such as watch
straps, cutlery etc.
• Titanium alloys have high strength, toughness and stiffness & are used in aerospace
structures .
Q,1 Why should we use alloys instead of pure metals?
Q.2 State uses of Aluminium & Stainless steel alloys.
1. What is meant by pure substance?
2. What is meant by mass percentage of solution?
3. Name the process of separation of miscible liquids.
4. Arrange the following in decreasing order of size of the particles.
True Solution , Suspension , Colloid.
5. *Give an example of an aqueous solution in which gas is dissolved.
6. Name the dispersion medium and dispersed phase in the white material inside an egg.
7. What happens when hot saturated solution is cooled?
8. How would you separate a mixture of chalk and water?
9. *How much water should be added to 15 grams of salt to obtain 15 % salt solution?
10. What type of mixtures are separated by technique of crystallization ?
1. Classify the following into elements, compounds and mixtures:
| a) Sodium | b) Soil | c) Sugar solution | d) Silver | e)Tin | f) Calcium carbonate | |
| g) Silicon | h) Coal | i) Air | j) Soap | k) Methane | l) Carbon dioxide | m) Blood. |
2. Give any two applications of centrifugation.
3. Which of the following is chemical change?
| a) Growth of a plant | b) Rusting of iron | c) Mixing of iron fillings and sand |
| d) Cooking of food | e) Digestion of food | f) Freezing of water |
| g) Burning of a candle. |
4. *State the difference between simple distillation & fractional distillation.
5. * A solution contains 40 ml of ethanol mixed with 100 ml of water. Calculate the
concentration in terms of volume by volume percentage of the solution.
1. *What is meant by Tyndall effect? What is its cause? Illustrate with example.
2. How would you separate the mixture containing sulphur and sand ?
3. What is crystallization? Give its two applications.
4. How are sol, solution and suspension different from each other?
5. How do we obtain coloured components, i.e. dye from Blue/Black ink ?
You are expected to know………
• Types of mixtures.
• Method of Separation of mixtures.
• Types of solutions.
• Concentration terms of solution.
• Physical and Chemical Change.
• Significance of alloys.
.png)