





Introduction
The branch of chemistry which deals with the identification of constituents of a substance and calculation of their amounts is called analytical chemistry.
Branches of Analytical Chemistry
The branches of analytical chemistry are
(a) Qualitative analysis It deals with the identification of various constituents present in a given material. It further be classified as
(b) Quantitative analysis It deals with the measurement of amounts/volume/strength of a substance/solution.
Qualitative Analysis of Inorganic Compounds
It involves the following steps
Preliminary Tests
(a) Solubility Compounds that dissolve in water to the extent of approximately 0.02 mol per litre (0.02M) are usually classified as “soluble” compounds, while those that are less soluble are classified as “insoluble” compounds. No gaseous or solid substances are infinitely soluble in water.
1. All common inorganic acids are soluble in water. Low molecular weight organic acids are soluble.
2. All common compounds of the group IA metal (Na, K etc) and the ammonium ion, NH4+, are soluble in water.
3. All common nitrates NO3– acetates, CH3COO– and perchlorates, CIO4COO–, are soluble in water.
4. (a) All common chlorides, CI– , are soluble in water except AgCl, Hg2Cl2 and PbCl2.
(b) The common bromides Br– , and iodides I–, show approximately the same solubility behaviour as chlorides. but there are some exceptions. As the halide ions (Cl– , Br–, I–) increase in size, the solubilities of their slightly soluble
compounds decrease. Although HgCl2 is readily soluble in water, HgBr2 is only slightly soluble and HgBr2 is even less soluble.
(c) The solubilities of the pseudo-halide ions, CN– (cyanide) and SCN– (thiocyanate), are quite similar to those of the
corresponding iodides. Additionally, both CN– and SCN– show strong tendencies to form soluble complex compounds.
5. All common sulphates, SO42-, are soluble in water except those PBSO4, and HgSO4.CaSO4and Ag2SO4 are sparingly soluble.
6. All common metal hydroxides are insoluble in water except those of the group IA metals and the lower members of the group IIA metals, beginning with Ca(OH)2.
7. All common carbonates CO32- phosphates, PO43-, and arsenates AsO43-, are insoluble in water except those of the group IA metals and NH4+.MgCO3 is fairly soluble.
8. All common sulphides, S2- are insoluble in water except those of the group IA and group IIA metals and the ammonium ion.
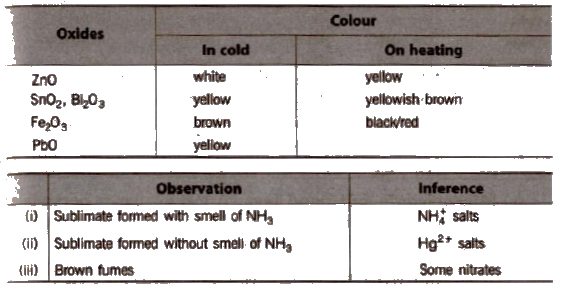
(b) Colour change on beating Certain oxides change colour on heating and this fact can be used to identify salt.
Dry Tests
(a) Borax bead test If borax, Na2B4O7·10H2O is heated on the platinum loop, a transparent colourless glass like bead of sodium metaborate (NaBO2) and boric anhydride B2O3) is formed.
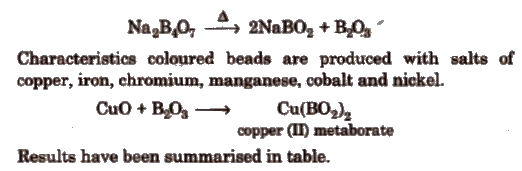
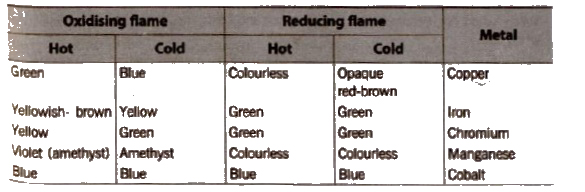
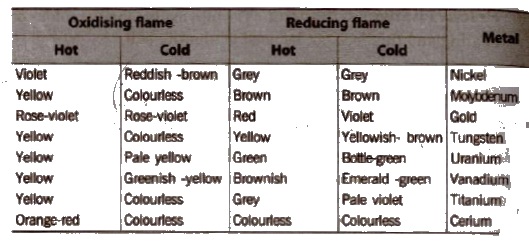
Colour In Oxidising and Reducing Flames In Borax-Bead Test
(b) Microcosmic salt bead test A test similar to borax bead test is used for identification of coloured cations if microcosmic salt, Na(NH4)HPO4·4H2O, is used.
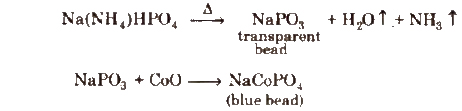
Results have been summarisod in the following table.
Microcosmic Salt Bead Test

(c) Sodium carbonate bead test The sodium carbonate bead is prepared by fusing a small quantity of sodium carbonate on a platinum wire loop in the Bunsen flame; a white, opaque bead is produced. If this is moistened, dipped into a littie KNO3 and then into a small quantity of a manganese salt (for example) and the whole heated in the oxidising flame, a green bead of sodium manganate (Na2MnO4) is formed.
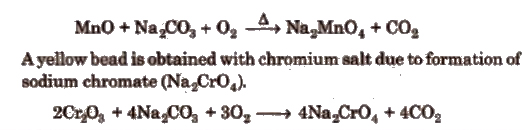
(d) Flame test Paste of the salt and cone. HCl is taken into the lower oxidising zone and colour imparted to the flame by salts is observed. Salts, particularly of group V (Ba2+, Ca2+, Sr2+) are identified by colours of the flame and summarised in Table
Flame Tests
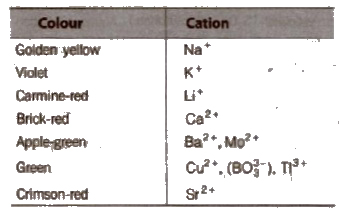
The yellow colouration due to sodium masks that of potassium. In such cases view the flame through cobalt glass, the yellow sodium colour is absorbed and the potassium flame appears crimson.
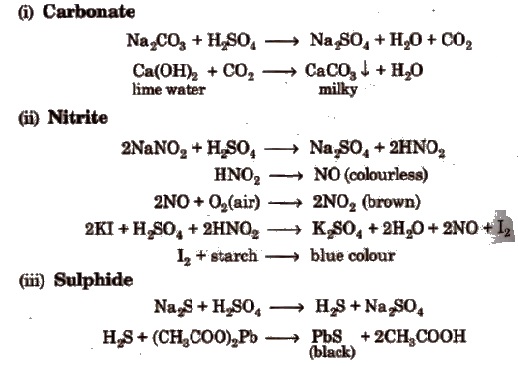
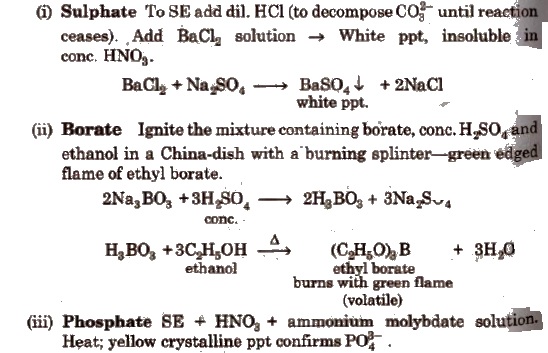
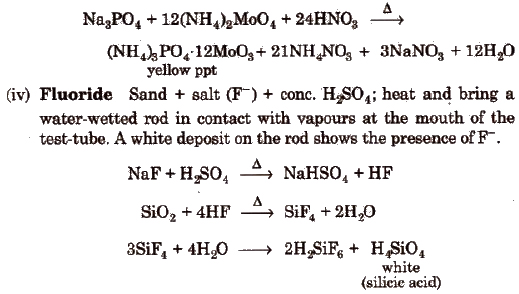
Wet Tests for Anions
Salt or mixture is treated with dil. H2SO4 and also with cone, H2SO4 separately and by observing the types of gases evolved, confirmatory tests of anions are performed.
Observation with Dilute H2SO4
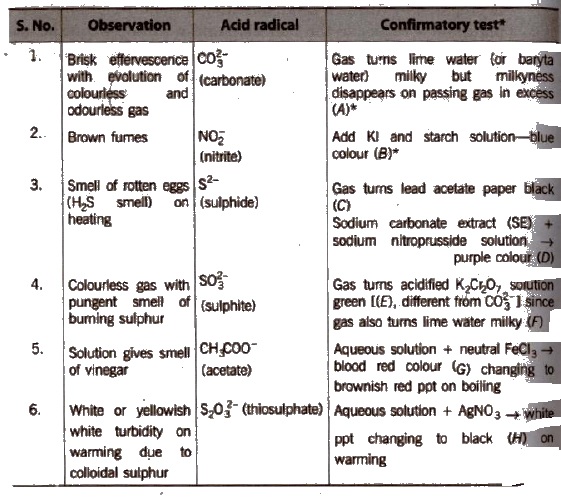

Observation with Concentrated H2SO4
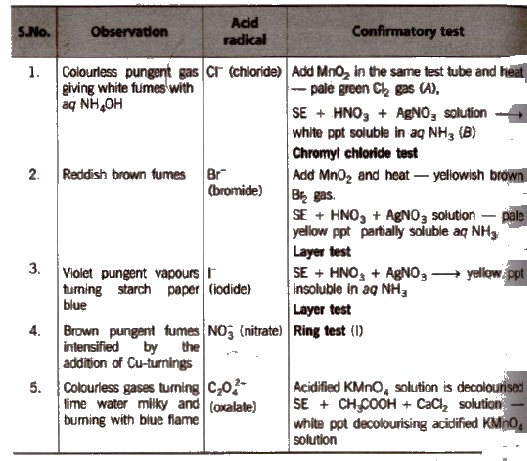
(i) Chloride
Chromyl-chloride test

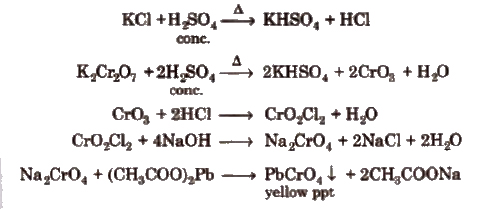
(ii) Bromide
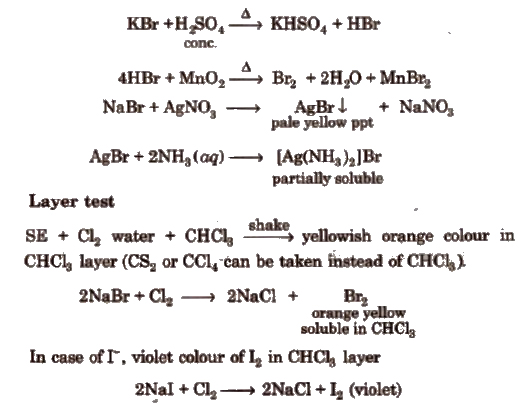
(iii) Iodide
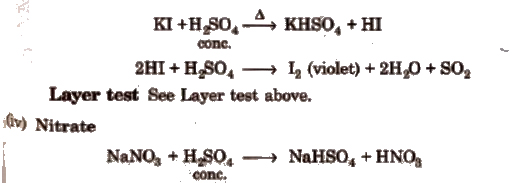

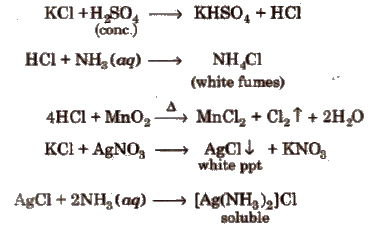
Wet Tests for Cations
Different basic radicals have different solubility products and precipitated by different reagents. Thus, on the basis of solubility products and reagent used, basic radicals are classified into following groups.
Classification of Basic Radicals Into Groups Based on k values
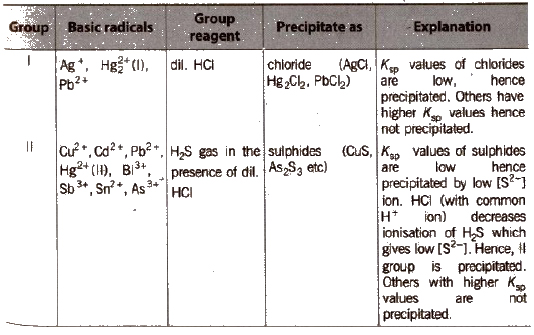
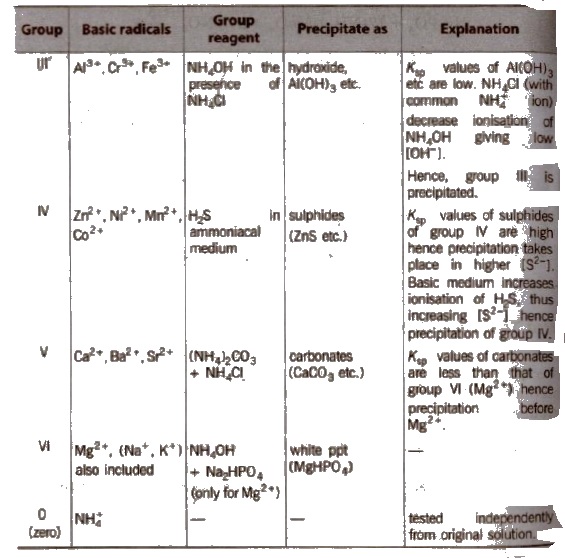
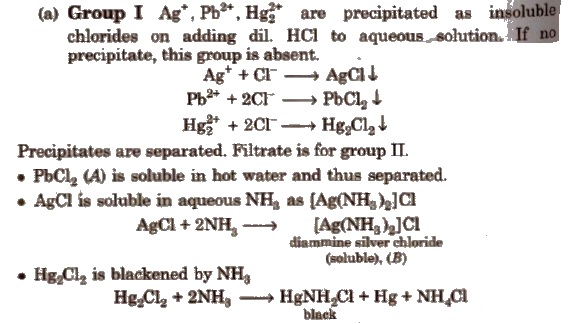

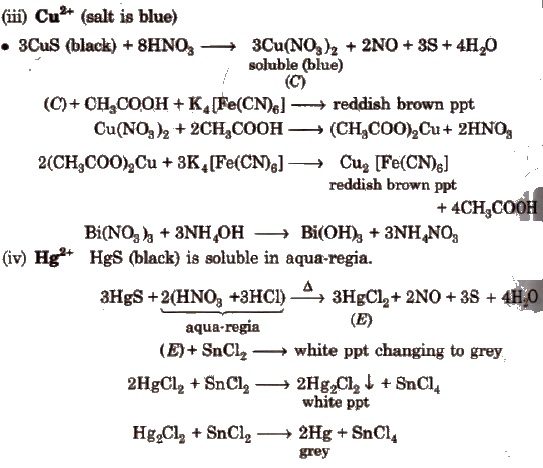
(c) Group III
Boil off H2S gas from the filtrate of group II. Add NH4Cl and one drop of dil. HNO3; heat, cool and add NH4OH → ppt
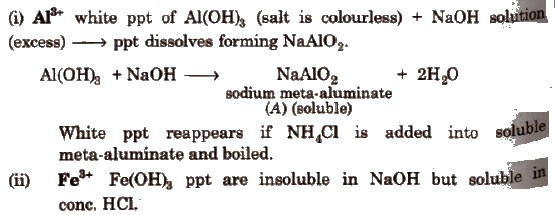

(d) Group IV
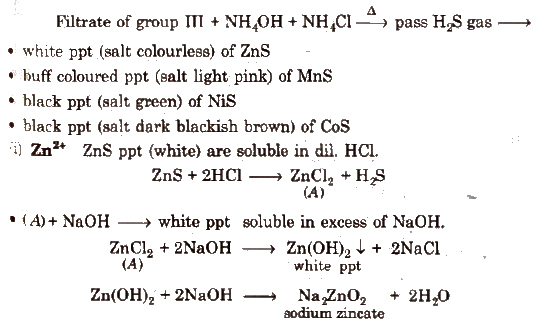
White ppt of Zns reappears on passing H2S into soluble Na2ZnO2 solution.
(ii) Mn2+ MnS (buff coloured ppt) are soluble in dil. HCl but addition of excess ofNaOH gives Mn(OH)2 ppt which changes to brown/black by atmospheric O2.
Dissolve MnO2 or Mn(OH)2 ppt into HNO3 and then add oxidising agent KClO3 or PbO2; heat to boil-purple solution (B).
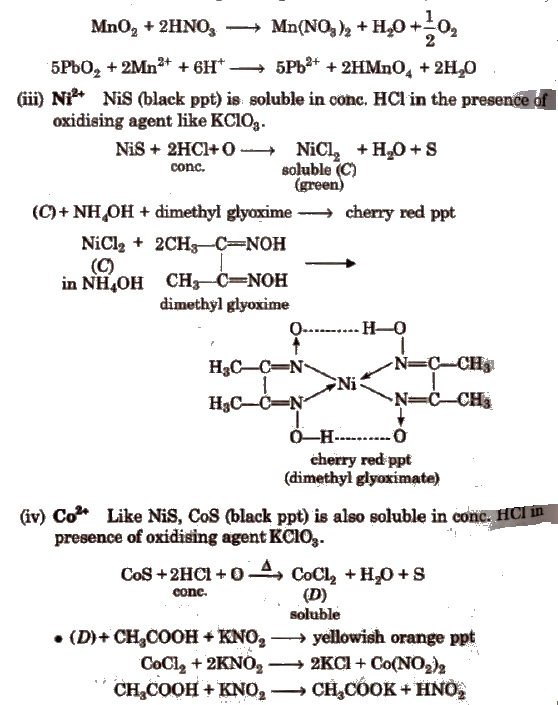
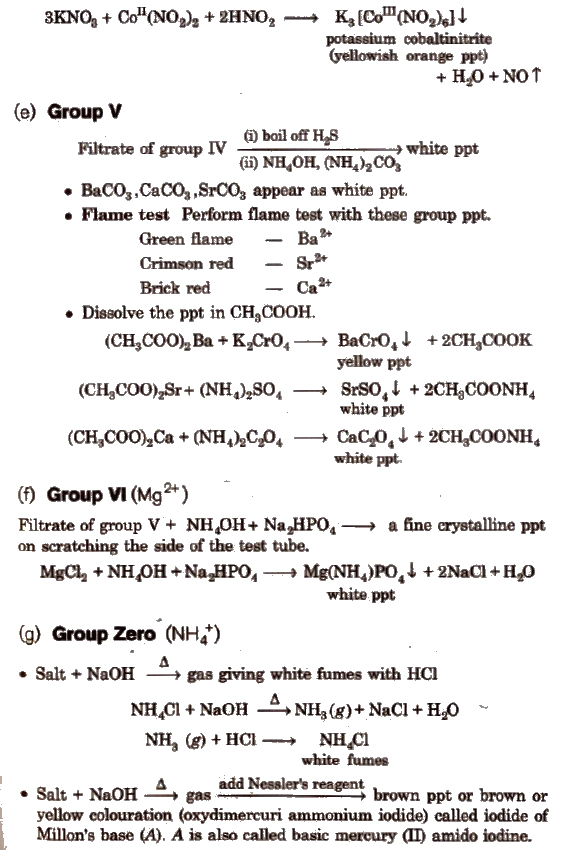
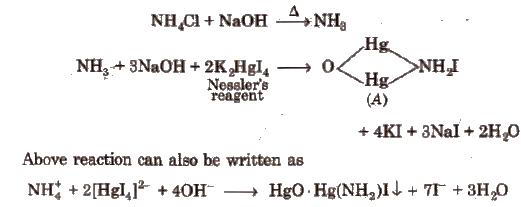
Some Other Important Tests
(a) Potassium Iodide (KI)

(c) K4 [Fe(CN)6] Potassium hexacyanoferrate (II) (Potassium ferrocyanide)

Qualitative Analysis of Organic Compounds
It also involves following steps:
1. Preliminary test
2. Element detection
3. Test of functional group.
1. Preliminary Tests
(a) Heat test The compound is heated in flame. If it burns with smoky flame, it is an aromatic compound and if it burns with
non-smoky flame, it is an aliphatic compound.
(b) Colour

(c) Odour
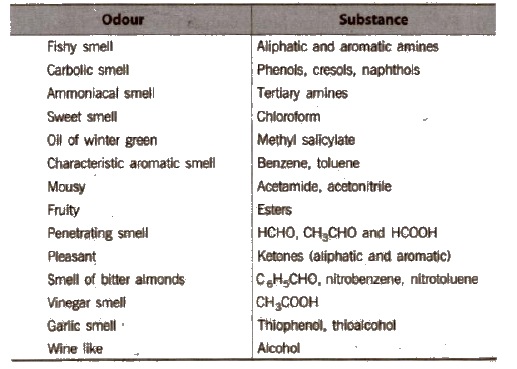
Element Detection
See chapter purification and characterisation of organic compounds.
Tests of Functional Groups
(a) Tests for Carboxylic (-COOH) Group
(i) Litmus paper test Dip blue litmus paper in the aqueous solution or suspension of the compound. It turns red.
(ii) Sodium bicarbonate test In a test tube take a little quantity of the compound and then, add a saturated solution of sodium bicarbonate. Formation of brisk effervescence shows the presence of -COOH group.
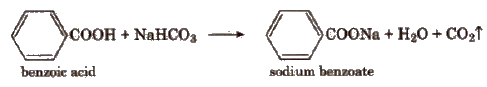
(iii) Ester formation Heat a small quantity of organic compound with ethyl alcohol and a little conc H2SO4. Cool the solution and pour in a tube containing water. A fruity smell, due to formation of an ester, indicates the presence of carboxylic group.

(b) Tests for Alcoholic (-OH) Group
(i) Certc ammonium nitrate test To small amount of organic compound or its aqueous solution, add a few drops of ceric ammonium nitrate. A red colour indicates the presence of alcoholic hydroxy group.
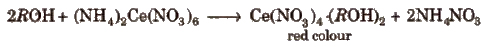
(ii) Lucas test This test is used to di stinguish between primary, secondary and tertiary alcohols. In this test, treat 2 mL of organic compound with about 8 mL of Lucas reagent (for preparing Lucas reagent dissolve 32 g of anhy ZnCl2 in 20 mL of cone HCl) and shake.
(a) Immediate formation of turbidity indicates the presence of tertiary alcohol.
(b) Formation of turbidity after 4-5 min shows the presence of secondary alcohols.
(c) If solution remains clear, then primary alcohol is present.
(c) Tests for Phenolic (Ph-OH) Group
(i) Ferric chloride test To aqueous or alcoholic solution of compound, add few drops of ferric chloride (FeCl a). Formation of green, blue or violet colour shows the presence of phenol.

(ii) Liebermann’s nitroso reaction Fuse a little amount of compound with a crystal of NaNO2 in a test tube. Cool the mixture and add 1 mL conc H2SO4.A deep green to blue solution is formed which turns red when poured in a large excess of water. The red aqueous solution becomes again deep green or blue if made alkaline with NaOH. It shows the presence of phenol.
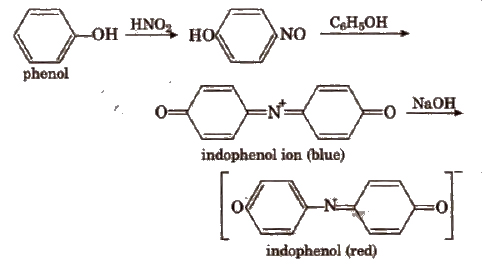
Note Nitrophenol does not respond to FeCl3 test as well as Liebermann’s nitroso reaction.
(d) Tests for Aldehyde (-CHO) Group
(i) Tollen’s reagent test Take a little quantity of the compound in a test tube and add 2 mL of freshly prepared reagent. Shake, warm and allow the contents to stand for 2-3 min. Formation of silver mirror or a grey ppt indicates the presence of an aldehydic group.
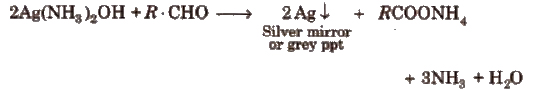
(ii) Fehling’s solution test Take a mixture of equal amounts of Fehling’s solution A and B, and a few drops of organic compound and boil the contents. Formation of a red ppt shows the presence of an aldehyde.
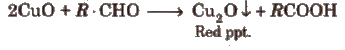
Note : Both the above test» are also given by reducing sugars.
(iii) Schiff’s reagent test Add 5-6 drops of organic compound to 2 mL of the reagent. Shake vigorously. Mer some time formation of a deep red or violet colour indicates the presence of an aldehydic group.
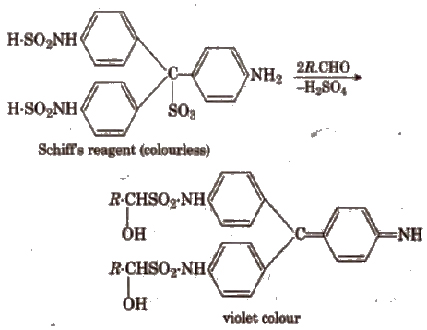
(iv) Benedict’s solution test Boil the compound with 2-3 mL of Benedict’s solution for few minutes. Appearance of a red-yellow ppt confirms the presence of aliphatic aldehydes.
Note : This test is usually given by only aliphatic aldehydes, thus used to differentiate between aliphatic and aromatic aldehydes.

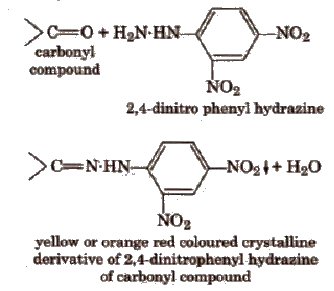
(i) 2,4-dinitro phenyl hydrazine test In a dry test tube add few drops of the organic compound (if liquid) or its alcoholic solution (if solid) to about 2 mL of the reagent and one drop of cone H2SO4. Shake vigorously, heat (if necessary) and allow to stand for about 5 min. A yellow or orange ppt separates out in case of a compound containing carbonyl group due to formation of respective hydra zones.
(ii) Sodium nitroprusside test Add 0.1 g of solid or 0.2 cc of liquid compound to 2 cc of sodium nitroprusside solution and then, make it alkaline with 2-3 drops of sodium hydroxide. A red or purple colour indicates the presence of ketone (benzophenone does not give this test).
(f) Tests for PrImary Amine (-NH2) Group
(i) Carbylamine test Boil a little quantity of the compound with 2 drops of chloroform and 2 mL of alcoholic caustic potash.
An intolerable offensive odour of carbylamine indicates the presence of primary amine.
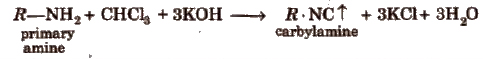
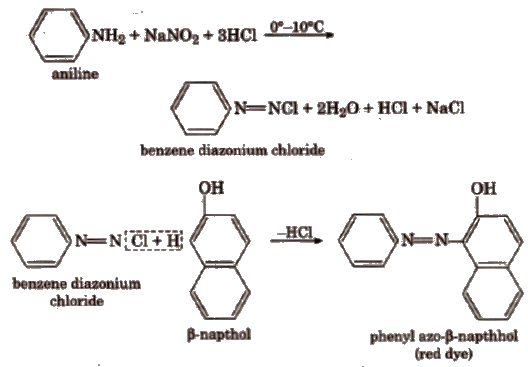
(ii) Dye test Dissolve about 0.2 g of the compound in dil HCl and cooL Now, add 10% aq NaNO2solution. Pour all this content into a beaker containing alkaline β-naphthol solution. Formation of a red or orange dye indicates the presence of aromatic primary ammo group.
(g) Tests to Distinguish between Primary, Secondary and Tertiary Amlnes
(i) Nitrous acid test Prepare a solution of nitrous acid by adding ice cold dil HCl to a solution of 1% aq NaNO2. Add gradually this solution to 0.2 g of the organic compound in 10 mL dil HCl.
(a) Formation of brisk effervescence shows the presence of aliphatic primary amine.
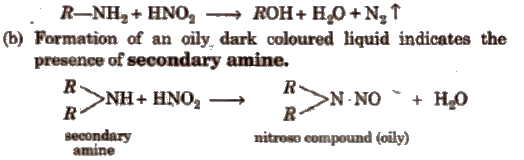
c) No reaction indicates the presence of aliphatic tertiary amine while production of green or brown colour indicates the presence of aromatic tertiary amines.
(ii) Hinsberg’s test To about 0.2 g of the compound) add 1 mL of 5% NaOH and 3 mL pyridine. Shake well and add few drops of benzene sulphonyl chloride with continuous shaking.
(a) Formation of yellow colour indicates the presence of primary amine.
(b) Formation of orange colour shows the presence of secondary amine.
(c) Formation of a red or purple colour shows the presence of tertiary amines.
Preparation of OrganiC Compounds
1. Acetanilide
Amines containing -NH2 and > NH groups respectively can be directly acetylated. Their reactive hydrogen atoms get replaced by the acetyl group (-COCH3) to give acetyl derivatives of the type RNH·COCH3 and R2N . COCHa respectively which may be regarded as mono and di-alkyl substituted acetamide.
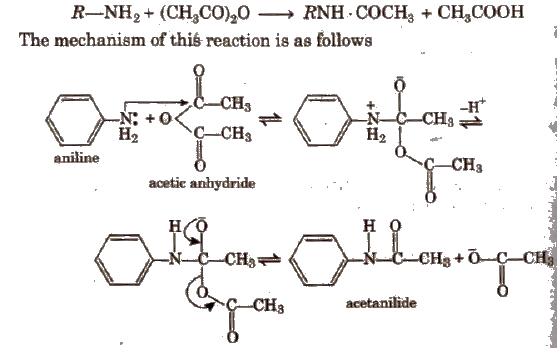
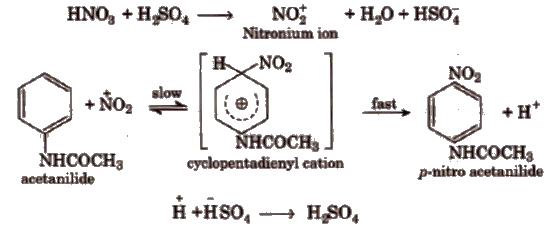
2. p-nitro Acetanilide
When acetanilide is treated with a mixture of cone HNO3 and conC H2SO4, it gives p-nitro acetanilide alongwith a little amount of o-isomer. In this process fuming HN0 3 in the presence of conc H2SO4 gives nitronium ion (NO4+) which attack on acetanilide to form p-nitro acetanilide through the cyclopentadienyl cation (intermediate) formation.
3. Aniline Yellow
When diazo amino benzene is heated with aniline and a little amount of aniline hydrochloride at about 40°C, for a short time, it gives p-amino azo benzene or aniline yellow in good yield.
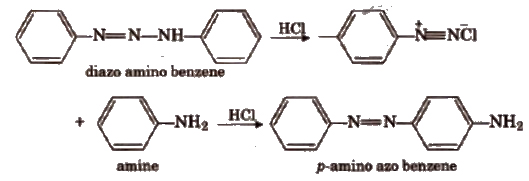
The mechanism of the reaction is based on the equilibrium involving the diazo amino compound, phenyl diazonium chloride and aniline.
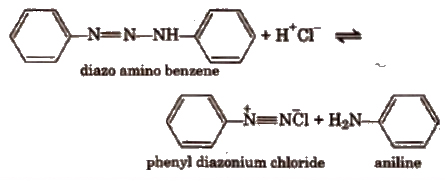
The reaction takes place between the two latter compounds under weakly acidic conditions.

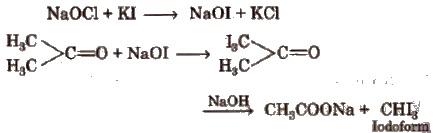
4. Iodoform
Acetone when treated with potassium iodide and sodium hypochlorite (NaOCl), gives iodoform.
Titrimetric Exercises
Some Important terms
(a) Titration The process by which the concentration or strength of a chemical substance is measured by using the solution of known strength is called titration.
(b) Analyte and titrant The substance being analysed is called analyte and that which is added to analyte in a titration is called titrant.
(c) Equivalence point or end point It is point at which the reaction between two solutions is just complete. It is generally represented by change in colour, pH, conductivity etc.
(d) Standard solution Solution of known concentration is called standard solution.
(e) Primary standard substance The substance standard solution of which can be prepared directly by dissolving its definite weight in definite volume of solvent is called primary standard substance, e.g., crystallie oxalic acid, anhydrous Na2CO3, Mohr’s salt etc.
The substance, which occur in pure state, are non-hydroscopic, non-deliquescent, generally behave as primary standard substance.
(f) Secondary standard substance Their standard solution cannot be prepared directly. e.g., KMnO4, NaOH, KOH etc.
(g) Indicator It shows the end point of a titration.
Na2CO3 vs HCl Titration
The titration of Na2CO3 us HCl is a neutralisation titration (acidimetry and alkalimetry) which involve the neutralisation of an acid with a base. e.g., sodium carbonate is attacked by dil HCl in the following way
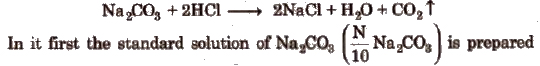
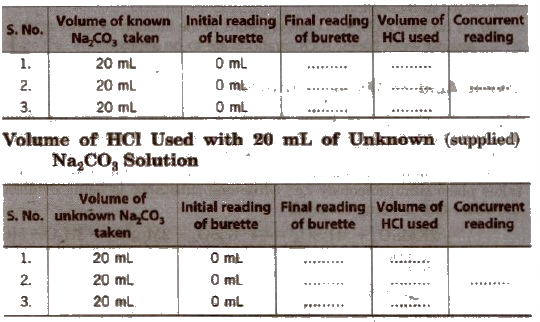
and then titrated its 20 mL with HCI solution by adding a few drops of methyl orange indicator. Change in colour shows the end point. Note the reading and repeat the procedure to obtain concurrent readings.
Volume of HCI Used with 20 mI, of Known (prepared) Na2CO3 Solution
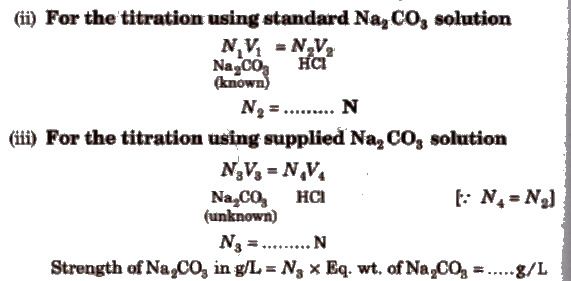
Calculations
(i) Weight of Na2CO3 dissolved in 250 mL measuring flask = z
= ….. g
Weight of Na2CO3 in 100 mL = ….. x 4
= ……. g/L
Normality of Na2CO3 (prepared) = [strength(g/L)/Eq. wt. of oxalic acid)
= (…… N/53)
Oxalic Acid vs KMnO4 Titration
This is an example of redox titrations, in which a reducing agent (as oxalic acid) is estimated by titrating it with a standard solution of oxidising agent (as KMnO4). Such reactions are accompanied by the change in valency of ions. In these titrations oxidation and reduction takes place simultaneously i.e., while one substance is being oxidised, the other one is being reduced.
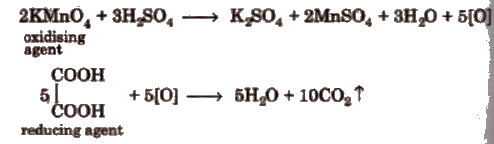
The last drop of KMnO4 itself acts as an indicator (self indicator).
In this titration, first the standard solution of oxalic acid is prepared which is than titrated KMnO4solution in the presence of dil. H2SO4. The procedure is repeated to obtain a set of concurrent readings.
Calculations
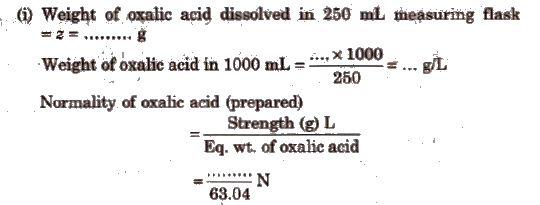
(ii) For the titrations using standard oxalic acid solution
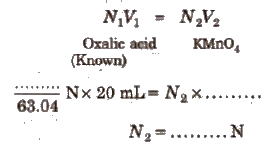
(iii) For the titration using supplied oxalic acid solution
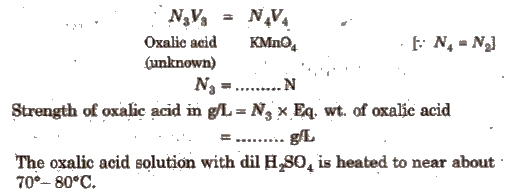
Sulphuric acid should be in excess otherwise a brown ppt due to formation of MnO2 will be formed.
This titration cannot be carried out in presence of acid like HNOs and HCI, because HNO3 itself is an oxidising agent, so it will interfere with the oxidising action of KMnO4 and HCl reacts chemically with KMnO4 solution.
Mohr’s Salt n KMnO4 Titration
This titration is also an example of redox titrations and work on the same principle as oxalic acid us KMnO4 titration. In this titration the active constituent of ferrous ammonium SUlphate (Mohr’s salt) is
ferrous sulphate, which is oxidised to ferric sulphate by acidified potassium permanganate as follows.
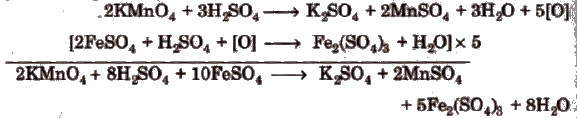
Calculations
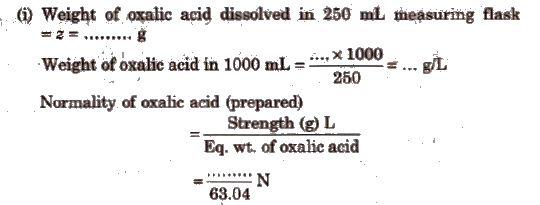
(ii) For the titrations using standard ferrous ammonium sulphate solution
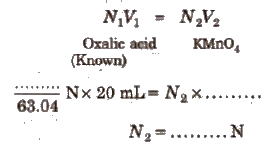
(iii) For the titration using supplied ferrous ammonium sulphate solution
N3V3 = N4V4
ferrous ammonium sulphate KMnO4 [because N4 = N2](unknown)
N3 = …N
Strength of ferrous ammonium sulphate in gIL = N3 x Eq. wt. of ferrous ammonium sulphate
= … g/L
.png)