





Organic compounds extracted from a natural source or synthesized in the laboratory requires purification. Various methods are used for the purification and are based on the nature of the compound and the impurity present in it. The purity of a compound is ascertained by determining its melting point or boiling point or by chromatographic and spectroscopic techniques.
Methods of Purification of Solids
Methods of Purification of Liquids
Chromatographic Method
It was discovered by Tswett (1906).
It is based upon the principle of selective adsorption of various components of a mixture between the two phases: stationary or fixed phase and mobile phase.The various chromatographic
techniques are:
1. Adsorption Chromatography
Stationary phase- Solid or ion exchange resin. Mobile phase-Liquid or gas.
It includes liquid-solid chromatography, gas-solid chromatography or ion exchange chromatography.
2. Partition Chromatography
Fixed phase-liquid supported on inert solid. Mobile phase-liquid or gas.
This process is known as liquid-liquid partition chromatography or liquid-gaspartition chromatography on the basis ofits different phases.
3. Paper Chromatography
The principle of paper chromatography is based on the fact that solutes have the capacity to migrate through filter paper at different rates as a solution is drawn into strip of paper by capillary action.
In paper chromatography, the dissolved substance is applied as a small spot about 2-3 cm from the edge of a strip or square of filter paper and is allowed to dry. This strip is then suspended in a large close container where atmosphere is saturated with the solvent system. The end containing the sample is dipped into the mobile pbase which has already been saturated with the stationary phase. When the solvent front has reached at the other end of the paper, the strip is removed and the zones are located by analytical methods.
(The ratio of the distance travelled by a component to the distance travelled by the solvent front is characteristic of each component and is known as the Rf value.
Rf = (distance in em from starting line to the centre of zone/distance in cm from starting line to the solvent front)
4. Column Chromatography
It is an example of adsorption chromatography. Adsorbents used are alumina, silica gel, cellulose powder, animal charcoal, keiselguhr etc.
Liquid solvents used are benzene. petroleum ether, alcohol etc.
When the solvent is poured over the mixture present at the top of a column packed with adsorbent, the components are separated into, number of layers called zones, bands or chromatograms due to preferential adsorption.
Elution The continuous pouring of solvent from the top of the column is known as elution or running of column. Solvent is known as eluant. The most weakly adsorbed component is eluted first by least polar solvent while more strongly adsorbed component is eluted later by highly polar solvents.
Chemical Methods of Purification
The substance to be purified is treated with a suitable chemical reagent to form a stable derivative. It is then separated by suitable method and decomposed to get the pure compounds.
Examples
1. Azeotropic Distillation
Azeotropes are constant boiling mixtures which distil off without any change in composition at a fixed temperature. Therefore, components of an azeotropic mixture cannot be separated by fractional distillation. A very common example of azeotropic mixture is rectified spirit which contains 95.87% ethyl alcohol and 4.23% water by weight which boils at 351.1 K.
(Such mixtures are separated by adding another component which generate a new lower boiling azeotrope that is heterogeneous (i.e., producing two immiscible liquid phases). e.g., C6H6 is added to H2O and ethyl alcohol azeotrope to separate them.
2. Differential Extraction
This method is used to separate an organic compound present in aqueous solution which is more soluble in other solvent than in water.
Qualitative Analysis of Organic Compounds
1. Detection of Carbon and Hydrogen
This is done by heating the given organic compound with dry cupric oxide in a hard glass test tube when carbon present is oxidised to carbon dioxide and hydrogen is oxidised to water.
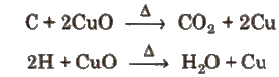
Carbon dioxide turns lime water milky.
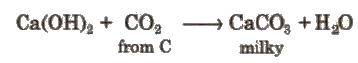
Water condenses on the cooler parts of the test tube and turns anbydrous copper sulpbate blue.

Lassaigne’s Test
The organic compound is fused with a small piece of Na metal. When
element (N, 8, X) of the organic compound combine to give NaCN,Na2S or NaX; the red hot tube is plunged in distilled water, boiled and filtered. The filtrate is called Lassaigne’s extract or sodium extract. The Lassaigne’s extract is usually alkaline. If not, it is made alkaline by adding a few drops of a dilute solution of sodium hydroxide.
The purpose of fusing the organic compounds with sodium metal is to convert halogens, N, S, P etc., present in the organic compound to their corresponding soluble sodium salts (ionic compounds).
Na + C + N → NaCN
2Na + S → N2S (where, X = Cl, Br, I)
Na + X → NaX
1. Detecton of Nitrogen
To a part of this alkaline solution is added a few drops of a freshly prepared solution of ferrous sulphate, because a dilute solution of FeSO4 after a long time oxidise to basic ferric sulphate which is useless for analysis. The contents are warmed a little, cooled and then acidified with dil. H2SO4. Appearance of a green or Prussian blue colouration indicates the presence of nitrogen.
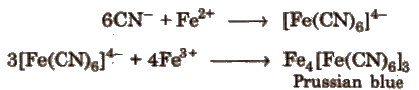
If S is also present alongwith N, a red colour in place of Prussian blue in the test of nitrogen appears, due to the formation of Fe(CNS)3.
Hydrazine does not give Lassaigne’s test for nitrogen since it does not contain carbon. In order to test the presence of N in such compounds, during fusion with Na, some charcoal or preferably strch (which contains C but not N, S, halogens etc.) is added. Under these conditions. C of starch or charcoal combines with N of the compound to form NaCN which will now give a positive test for nitrogen.
Lassaigne’s test is not shown by diazonium salts because diazonium salts usually lose N2 on heating much before they have a chance to I react with fused sodium metal.
2. Detection of Sulphur
(i) Sodium fusion extract is acidified with acetic acid and acetate is added to presence of sulphur.
(ii) On treating sodium fusion extract with sodium nitroprusside, apperance of a violet colour further indicates the presence of sulphur.
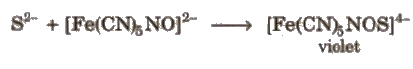
3. Detection of Halogens
The sodium fusion extract is acidified with nitric acid and then treated with silver nitrate.
X– + Ag+ → AgX
X represents a halogen -Cl Br, or I.
AgCl white ppt, AgBr-dull yellow ppt, AgI-bright yellow ppt.
Note Beilstein test is also a test for halogen but it is not a confirmatory test.
Detection of Phosphorus
The compound is heated with an oxidising agent (sodium peroxide). By this the phosphorus present in the compound is oxidised to phosphate.
The solution is boiled with nitric acid and then treated with ammonium molybdate. A yellow colouration or precipitate indicates the presence of phosphorus.
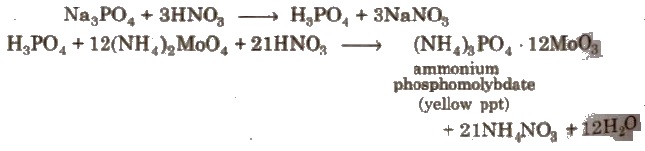
Detection of Oxygen
There is no direct method to detect oxygen in compounds. It is present in the form of functional groups such as -OH, -COOH. -NO2 etc.
Qauantitative Estimation of Elements
1. Estimation of Carbon and Hydrogen (Liebig’s Method) When a known mass of organic compound is strongly heated with dry euO, C and H present are quantitatively oxidised to CO2and H2O respectively,
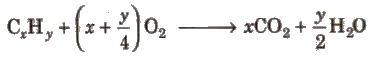
By knowing the amount of CO2 and H2O from known weight of organic compound, the percentage of carbon and hydrogen can be computed,
The water is absorbed in anhydrous CaCl2.
The carbon dioxide is absorbed in concentrated solution of KOH.
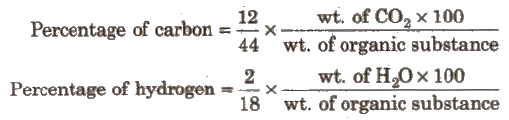
.
On heating with CuO, elements other than C and H are also modified as follows:
When organic compound contains nitrogen. the oxides of nitrogen
(NO,N2O etc.) are absorbed by caustic potash. These are removed by the use of bright copper gauge.
4Cu + 2NO2 → 4CuO + N2
Cu + N2O → CuO + N2
Nitrogen is not absorbed by KOH solution.
When organic compound contains halogens, they are removed by using silver gauge by forming non-volatile silver halide.
When sulphur is present, it is removed by forming lead sulphate by using fused lead chromate and halogens form lead halides.
Estimation of Nitrogen
(i) Duma’s method This method is used for nitrogenous compounds. Though tedious but it is better than Kjeldahl’s method.
In this method, the nitrogenous compound is heated strongly with ceo in the atmosphere of CO2and the mixture obtained is passed over a roll of heated bright Cu gauze. The oxides of nitrogen again reduce to N2. The resultant mixture is passed in KOH. All gases except N2 are fairly absorbed. Nitrogen is collected over KOH and its volume at NTP is measured.
Organic compound + conc. H2SO4 + (small amount of K2SO4 and its volume at NTP is measured.
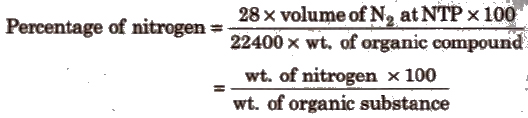
(ii) Kjeldahl’s method Organic compound + conc. H2SO4 + (small amount of K2SO4 and
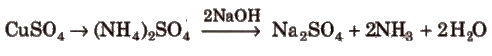
Ammonia is passed through H2SO4 or HCl of known volume and normality. The volume of acid neutralised by NH3 is calculated by neutralising the acid left by NaOH solution.
Percentage of nitrogen = (1.4 x N x V/wt. of organic compound)
N = normality of acid
V = volume of acid in mL neutralised by ammonia.
(In practice, K2SO4 is added to raise the boiling point of H2SO4 and CuSO4 is added to catalyse the reaction).
Kjeldahl’s method is not reliable as results obtained are generally low. It cannot be applied to compounds containing nitrogen directly linked to oxygen or nitrogen such as nitro, nitroso, azo and nitrogen present in ring as in pyridine.
Estimation of Halogen (Carius Method)
Organic compound + fuming HNO3 + AgNOa → AgX
It is estimated gravimetrically.
Percentage of halogen = (wt. of halogen atom x wt. of AgX x 100/mol. wt. of AgX x wt. of organic compound)
Estimation of Sulphur

Estimation of Phosphorus
Organic compound + Fuming nitric acid → H3PO4
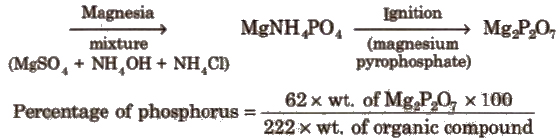
Now a day CHN elemental analyser is used to estimate the C, H and N in the organic compound.
Determination of Empirical Formula
Empirical formula expresses the relative number of atoms present in the molecule. It is calculated from percentage composition of the compound.
Determination of Molecular Formula
Molecular formula = (Empirical formula)n
n = (molecular weight/empirical formula weight)
.png)