





A colloid is a heterogeneous system in which one substance is dispersed (disperse phase) as very fine particles in another substance called dispersion medium. The study of the colloidal state of matter was started by Thomas Graham (1861).
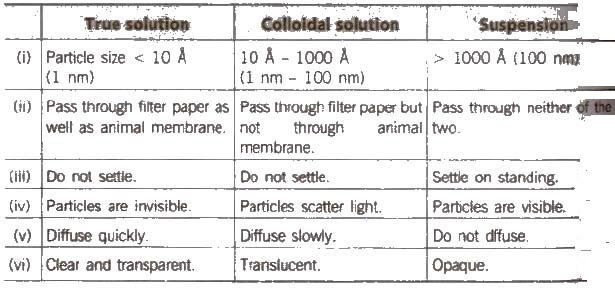
Comparison of True Solution, Colloidal Solution and Suspension
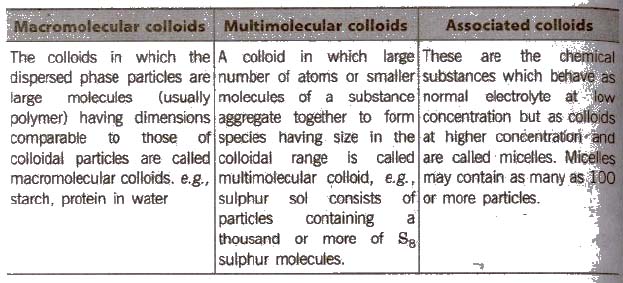
Classification of Colloids
(A) Types of colloids based on physical state of dispersed phase and dispersion medium
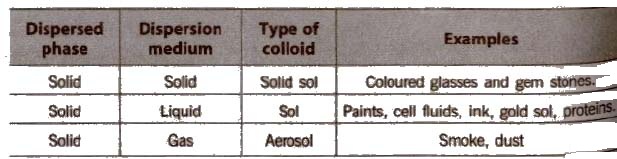
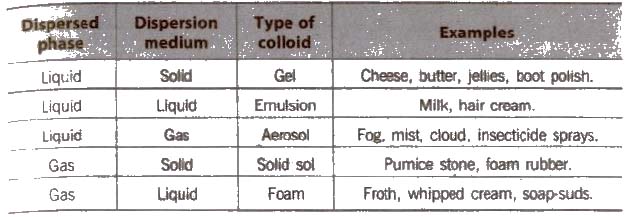
Depending on the nature of dispersion medium. the colloids can be named as hydrosols or aquasols (for water), alcohols (for alcohols), benzosols (for benzene) and aerosols (for gases),
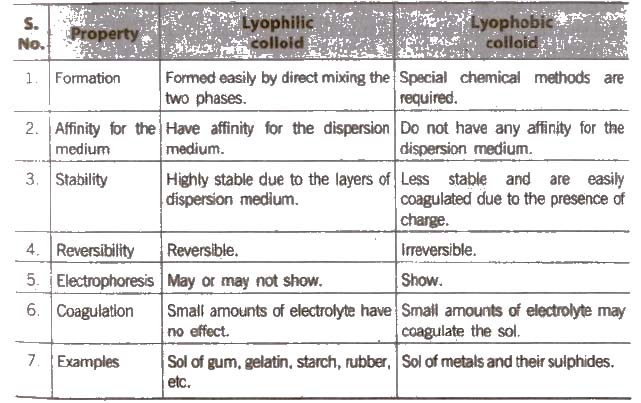
(B) Types of colloids based on nature of interaction between dispersed phase and dispersion medium
(C) Types of colloids based on type of particles of the dispersed phase
Kraft temperature (Tk) It is the minimum temperature of the colloidal system above which the formation of micelles takes place.
Critical micelle concentration (CMC) The minimum concentration of the surfactant at which the formation of a micelle takes place is called critical micelle concentration, e.g., CMC for soaps is ~ 10-4 to 10-3 mol L-1.
Preparation of Colloids
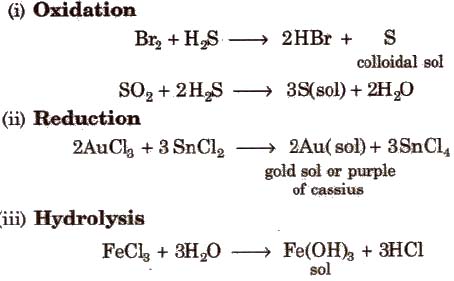
Condensation / Aggregation Method
These methods involve the joining of a large number of small particles to form particles of colloidal size. Some methods are
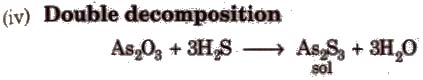
Dispersion/Disintegration Method
In this method, bigger particles are broken down to colloidal size. Some methods are
(i) Mechanical disintegration In this method, suspension is ground well in a colloid mill consisting of two steel discs which rotate in opposite directions at very high speed, to obtain the particles of colloidal size.
(ii) Electrical disintegration/Bredig’s Arc method In this method, electric arc is struck between electrodes of the metal (gold, silver, platinum, etc) immersed in the dispersion medium. The intense heat produced vapourises the metal which then condense to form particles of colloidal size.
(iii) Peptization This method is used to convert fresh precipitate into colloidal state by shaking with dispersion medium in the presence of small amount of electrolyte. The electrolyte used for this purpose is called peptizing agent.
Purification of Colloidal Solutions
The process used for reducing the amount of impurities to a requisite minimum of a colloid, is known as purification of colloidal solutions.
(i) Dialysis It is based upon the principle that impurities of true solutions can pass through the parchment paper or cellophane membrane while colloidal particles cannot.
In this process, dissolved substances are removed from the colloidal solution by means of diffusion through a suitable membrane.
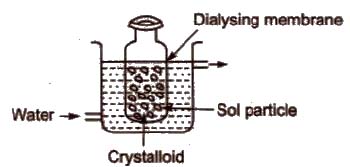
(ii) Electro-dialysis The process of dialysis is quite slow. So,if the dissolved substance in the impure colloidal solution is only the electrolyte then electric field is applied. The colloidal solution is placed in a bag of suitable membrane, while pure water is taken outside.
(iii) Ultrafiltration Ultrafiltration is the process of separation of colloidal particles from the solvent and soluble solutes present in the colloidal solution by specially prepared filters, called ultrafilters.
Properties of Colloidal Solution
General Properties
(i) Colligative property Due to high average molecular masses of colloidal particles, mole fraction of the dispersed phase is very low. So, the values of colligative property are very small.
(ii) Colour The colour of colloidal solution depends on the wavelength of light scattered by the dispersed particles. The wavelength of light further depends on the size and nature of the particles. The colour of colloidal particles also depends on the manner in which the observer receives the light.
(iii) Visibility The particles of colloidal solution are not visible to naked eye or under ordinary microscope.
(iv) Filterability Colloidal particles can pass through ordinary filter papers, but can’t pass through parchment paper or animal membrane.
Optical and Mechanical Properties
(i) Brownian movement Sol particles move in a random zig-zag manner due to the unequal impacts of the particles of dispersion medium on the particles of colloidal sol. It is called Brownian motion. Smaller the size of the particle and lesser the viscosity of the solution, faster is the motion.
(ii) Tyndall effect If a colloidal solution is placed in dark and a beam of light is passed through the sol, the path of light becomes visible with a bluish light. This phenomenon is called Tyndall effect. The scattering of light illuminates the path of beam in the colloidal dispersion.
Tyndall effect is observed only when the following two conditions are satisfied:
(i) The diameter of the dispersed particles is not much smaller than the wavelength of the light used.
(ii) The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude.
Tyndall effect is also observed when sunlight enters in a dark room through a slit or when light is thrown from a light projector in a cinema hall. Tale of comets is seen as a Tyndall cone due to scattering of light by the tiny solid particles. left by the comet in its path.
Electrical Properties
The charge on the particles is due to either the given reasons
Positively charged colloids are metal hydroxides, basic dyes like methylene blue sol. Protein in acidic medium. oxides like TiO2 sol. Examples of negatively charged colloids are metals (like Cu. Ag, Au. etc.) metal sulphide. acid dyes like eosin and sols of starch, gum. gelatin. clay. charcoal, etc.
It can be brought about by :
Coagulating value is the minimum amount of electrolyte (in millimoles/litres) needed to coagulate the colloidal solution. Smaller the coagulating or flocculating value of an electrolyte, greater is its coagulating power.
Coagulating power ∝ 1 / Flocculating value
Hardy-Schulz rule Greater the valency of the oppositely charged ions of the electrolyte, more will be its coagulating power, i.e. coagulating power ∝ charge of ion, e.g., for As2 O3 sol the order is

Protective Colloids
In the presence of a lyophilic colloids, lyophobic sol gets protected towards the action of electrolyte This phenomenon is called protection and the lyophilic colloid is termed as protective colloid.
Gold Number
The protective power of protective colloid IS measured in terms of gold number which is defined as the number of mg of the protective colloid which just prevents the coagulation of 10 ml, of standard gold sol when 1 mL of 10 % solution of NaCl is added to it.
[Smaller the gold number of a protective colloid, greater is its protective power.]
Gold number of gelatin is 0.005 – 0.01 and oi starch is 20·25.
Emulsion
It is a colloidal dispersion in which both dispersed phase and dispersion medium are liquid.
Types of Emulsions
Dye test and dilution test must be used to distinguish between the two types of emulsions.
Emulsifiers
Emulsifying agents or emulsifiers are the substances added in small quantity to stabilize the emulsions of fairly high concentration.
Demulsification The separation of an emulsion into its constituent liquids is called demulsification. It can be carried out by freezing boiling. centrifugation, etc
Gels
Gel is a liquid-solid colloidal system in which a liquid is dispersed in a solid.
Gels are of two types: elastic gels e.g. gelatin, agar-agar, starch) and non-elastic gels (e.g., silica. alumina and ferric oxide).
When gels are allowed to stand. they give out small quantity of trapped liquid and the gel shrinks in volume. This phenomenon is called syneresis or weeping of gel.
Applications of Colloids
.png)