





Heating Effects of Current
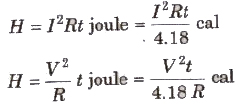
When current 1 flows through a conductor of resistance R for a time t then heat generated in it is given by
where, V = potential difference applied across the end, of the conductor.
Electric Power
The electrical energy produced or consumed per unit time is called electric power.
or
The rate at which work is done by the source of emf in maintaining the electric current in a circuit is called electric power of the circuit.
Electric Power, P = VI = I2R
= (V2/R)
where, V is the potential difference across the conductor, I is flowing through the conductor and R is the resistance.
Its SI unit is watt (W).
The other units of electric power are kilowatt and horse power.
Electric Energy
The energy supplied by any source in maintaining the current in the electric circuit is called electric energy consumed by the electric circuit.
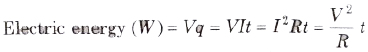
Its SI unit is joule (J) but another unit is watt-hour. The bigger unit of electric energy is kilowatt hour (kWh). It is known as board of trade (SOT) unit.
1 kilowatt hour = 1000 watt x 1 hour
= 1000 J/s x 3600 s
= 3.6 x 106 J
1 Horse power = 746 watt
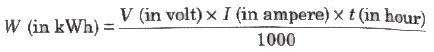
The electric energy consumed in kWh is given by
Electric Fuse
An electric fuse is a safety device used for protecting electric circuits bum demaging it due to excess flow of current. It is made of tin-lead alloy (63% tin + 37% lead).
It should have high resistance and low melting point and should be connected in series with the live wire.
Maximum safe current which can be passed through a fuse wire is it dependent of its length. However, it depends on the radius r of wire as
I ∝ r(3/2)
Short Circuiting
When accidentily the live wire comes in contact with nutral wire, resistance of the circuit decreases and a high current flows through circuit. This phenomena is called short circuiting.
Overloading
When a high current flows through the wire which is beyond the ra of wire, then heating of wire takes place. This phenomena is ca overloading.
Rating of Electrical Appliances
The values of power and voltage taken together for an el appliance is called rating of the appliance.
Fusing of Bulb When it is Switched On
Usually filament bulbs get fused when they are switched on. This is because with rise in temperature the resistance of the bulb increases and becomes constant in steady state. So, the power consumped by the bulb (V2R) initially is more than that in steady state and hence the bulb glows more brightly in beginning and may get fused.
Chemical Effect of Electric Current
When a direct current flows through a acidic or basic solution it dissociate into positive and negative ions. This phenomena is called electrolysis and these liquids are called electrolytes.
Some Terms of Electrolysis
Faraday’s Laws of Electrolysis
First Law
The mass of the substance liberated at each electrode is directly proportional to the total charge passed through the electrolyte.
m ∝ q
Or M = Zq = ZIt
Second Law
The mass of each substance liberated at the electrodes in electrolysis by a given amount of change is proportional to the chemical equivalents of the substances.
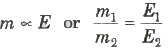
where m1 and m2 are masses of the substance liberated on electrodes by passing same amount of current for the same time and E1, E2 are chemical equivalents of these substances.
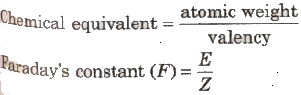
Faraday’s constant is equal to the amount of charge required to liberate one chemical equivalent (in gram) of mass of a substance at a electrode during electrolysis.
lta value is 96500 C/g equivalent.
Faraday’s constant, (F)= Ne
where N = Avogadro’s number and e = electronic charge.
Electroplating
The process of coating an object, that conduct electricity, with another metal is called electroplating. The article of cheep metal are coated with precious metal like silver and gold to make them look attractive,
Anodising
The process of coating aluminium with its oxide electrochemically to protect it against corrosion is called anodising.
Electrolysis is used for local anaesthesia. For it current is passed through a nerve, due to which it becomes insensitive to pain. Electrolysis is used for nerve stimulation of polio patients.
Electrochemical Cell
An electric cell is a device which maintains a continuous flow of charge in a circuit by a chemical reaction.
1. Primary Cell
Primary cell is an electrochemical cell, which once discharge cannot be put to use again by passing electric current from an external source.
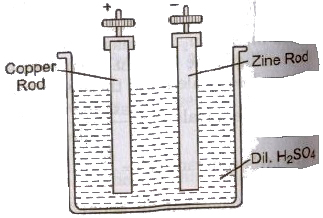
(i) Voltaic This cell was first discovered in 1791 by scientist Galvani and Copper 1 i Zin:Rod 1800 by scientist Volta. To set up a simple cell, take a glass vessel containing dilute sulphuric cell, acid as the electrolyte. Two rods, one of copper and the other of zinc are kept immersed in the electrolyte. These rods are kept separated from each other. The” are called the electrodes. Due to chemical reaction a potential difference of 1.08 V is developed between these rod.
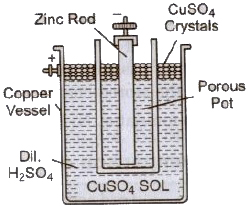
(ii) Dainel Cell It consists of a Zinc Rod copper vessel in which a porous pot containing dilute sulphuric acid is placed. An amalgamated zinc rod (which acts as the negative electrode) is put in the porous pot. The copper vessel contains a Dii. concentrated solution of copper sulphate which acts as an oxidising agent. To maintain the strength of copper sulphate solution, crystals of the copper sulphate are placed on the perforated shelf, partly immersed in the solution.
(iii) Leclanche Cell In this cell, anode is a carbon rod placed in a porous pot containing a mixture of powdered carbon and manganese dioxide. The carbon powder is used to make the manganese dioxide conducting or to increase the surface area of the carbon electrode. An amalgamated zinc rod is used as a negative electrode.
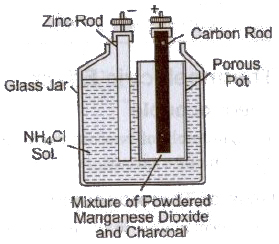
(iv) Dry Cell It is modified form of a Leclanche cell. The electrolyte in a dry cell is in the form of a jelly composed of starch, flour and ammonium chloride. The positive electrode is a carbon rod surrounded by a mixture of manganese dioxide and carbon contained in a cloth bag. This bag is placed in a zinc can and the space in between is filled with the ammonium chloride jelly. The zinc can itself acts as the negative electrode.
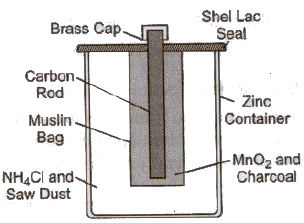
2. Secondary Cell
Secondary cell is an electrochemical cell, which has to be char initially by passing electric current from an external source, so as convert the stored chemical energy into electrical energy.
Lead accumulator, Ni-Fe or alkali accumulator are the secondary ce
Comparison of lead accumulator and Ni-Fe cell
| S.No. | Lead Accumulator | Ni-Fe cell |
| (i) | It can be used to draw strong current. | It can not be used to draw strong current. |
| (ii) | When it is completely charged its emf is 2.2 V. | When it is completely charged its emf is 1.35 V. |
| (iii) | Its efficiency is nearly 70%. | Its efficiency is nearly 50%. |
Thermoelectric Effect
Thermocouple
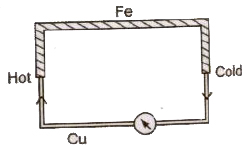
The assembly of two different metals joined at their ends to have junctions in a circuit, is called a thermocouple.
Uses of Thermocouple
Seebeck Effect
When the two junctions of a thermocouple are kept at different temperatures, then an emf is produced between the junctions, which is called,thermo emf and this phenomena is called Seeback effect.
Thermoelectric Series or Seeback Series
Seeback form a series of metals for which thermoelectric current flows a thermocouple through the hot junction from a metal occuring earlier, to a metal occuring later, in series.
The series is given below
Bi, Ni, Co, Pd, Pt, Cu, Mn, Hg, Pb, Sn, Au, Zn, Cd, Fe, Sb, Te
Neutral Temperature (Tn)
The temperature of the hot junction at which thermo emf in a thermocouple is maximum, is called neutral temperature.
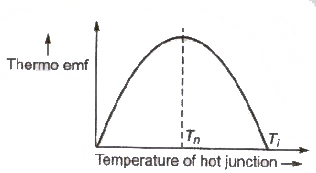
Temperature of Inversion ( Ti)
The temperature of the hot junction at which the thermo emf in a thermocouple becomes zero and beyond it, reverses its direction is called temperature of inversion.
Its value depends upon the temperature of the cold junction as well as the nature of the materials forming the thermocouple.
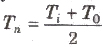
Relation between neutral temperature (T„) and temperature of inversion ( Ti) is given by
Where T0 is the temperature of cold junction.
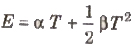
When cold junction is at 0°C, then thermo emf changes with temperature of hot junction (T) at follows.
where α and β are constants depending upon nature of metals forming thermocouple.
Thermoelectric Power
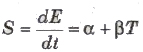
The rate of change of thermo emf with temperature, thermoelectric power.
Thermoelectric Thermometer
It is a device used to measure both low and high temperatures. Thermoelectric thermometer have much wider range of measurement of temperature (from —200°C to 1600°C). They are quite sentitive and can measure temperature accurately upto 0.05°C. Disadvantage of thermometer is that it does not give direct reading and hence it cannot be used in experiments on calorimetry.
Peltier Effect
Peltier Coefficient
The ratio of heat energy absorbed or evolved at a junction of a thermocouple to the charge flowing through it called Peltier coefficient.
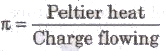
Peltier coefficient,
Its SI unit is JC-1‘.
Thomson’s Effect
If two parts of a single conductor are maintained at different temperatures, then an emf is produced between them, which is calico Thomson’s emf and this phenomena is called Thomson’s effect.
Thomson’s Coefficient
The ratio of Thomson’s emf and temperature difference between two points is called Thomson’s coefficient.
Thomson’s coefficient, (σ) = (dV/dT)
where, dV = potential difference between two points and dT = temperature difference between the two same points.
Thermopile
It is a combination of a large number of thermocouples in series. A’ such, it is able to detect the heat radiation and to note small variation or difference in temperature.
.png)